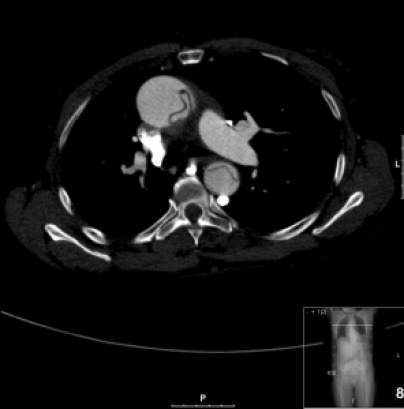
A 67 year-old male with history of hypertension, diabetes mellitus type II, dyslipidemia presents to the emergency department with 1 hour of severe chest discomfort that started while he was watching the evening news at home. The chest discomfort is substernal and radiates to his left shoulder and back. Review of symptoms is positive for nausea, sweats, and mild lightheadedness. Vital signs: BP (right upper extremity) 186/114 (left upper extremity) 168/98, HR 98, RR 21, T 98.6, SpO2 99%, FSG 136 mg/dl. The patient appears to be uncomfortable and anxious, mildly diaphoretic, and is clutching his chest. The cardiovascular exam is otherwise unremarkable. As part of your evaluation the following image is obtained:
Questions:
1) What are the alarming symptoms and signs in this patient's history and physical exam?Chest pain/discomfort in the emergency department is a high-risk chief complaint in and of itself, such that patients presenting with this complaint deserve your prompt attention. The differential diagnosis for chest pain and/or discomfort is broad and includes immediately life- and limb-threatening diagnoses such as acute coronary syndromes, acute aortic syndromes, pneumothorax, pulmonary embolism, esophageal rupture. Therefore, in the patient who is not “coding” in front of you and requiring ACLS protocols, it is critical to elicit an accurate and meaningful history and physical examination consisting of truly pertinent positive and negative findings.
This patient, for example, is in his late 60s, is male, and is presenting with acute onset chest pain, all high risk features. In fact, this patient is, at the very least, at risk for all the aforementioned diagnoses. His symptoms are further characterized by radiation to the back and left shoulder, with accompanying lightheadedness. The examination findings demonstrating an upper extremity brachial blood pressure differential is also very concerning and very, very pertinent to look for. This raises concern for possible acute aortic dissection in the setting of acute, severe chest pain as the chief complaint, but does not quite rule it in as a final diagnosis. Finally, this patient has a high risk past medical history which is extremely pertinent: Hypertension.
Together, in the setting of acute onset chest pain, these historical and physical findings raise concern for possible acute aortic dissection. This is a DO NOT MISS diagnosis with a mortality of ~75% at 2 weeks and 1-3% per hour untreated within the first 48 hours (1).
The pain and exam features and predisposing conditions are useful in assessing the risk for aortic dissection; they have some pre-test probability. High risk pain features include: Abrupt in onset, severe chest pain described as ripping, tearing, or stabbing in quality. High-risk exam findings include: Blood pressure differentials, pulse deficits or differences in quality, hypotension or shock, new or acutely worsening heart murmurs characteristic of aortic regurgitation, and any combination of focal neurological deficits (1,2). High-risk predisposing medical conditions include Marfan Syndrome, large vessel vasculitis and other mixed connective tissue diseases, aortic valve disease/repair, bicuspid aortic valve, cocaine or MDMA abuse, and history of hypertension among other (1,2).
Why is all of this important? When you encounter a patient the next time with chest pain, ask yourself if you can be sure that this patient is not currently having an acute aortic dissection. If you cannot, then make sure to elicit pertinent history and exam findings, and appropriately document so that you and readers of your note can sigh in relief that the risk of acute aortic dissection is indeed very, very low. Otherwise your work is not done!
Several studies have attempted to look at the features that raise the pre-test probability of acute aortic dissection (1-8). Although there is no one specific feature that can solidify the diagnosis or completely rule it out there are clusters of features that together either lower or increase your pre-test probability of acute aortic dissection and therefore justify further diagnostic imaging. For example:
- If the pain is not sudden in onset: Neg LR=0.3 (95% CI 0.2-0.5). So make sure you really get a good story about when and how the chest pain started (2).
- Pulses that are appreciably different on palpation increase the likelihood of acute aortic dissection by a significant degree with a Pos LR=5.7 (95% CI 1.4-23) (1,2).
- Be sure to include a thorough neurological exam because the presence of focal neurologic deficits increases the likelihood of acute aortic dissection significantly with a Pos LR=6.6-33.0 (1,2).
- Although no radiograph is mentioned in the above vignette, considering the presence of mediastinal widening on a plain film in the setting of a patient presenting with acute-onset “aortic-type” chest pain with pulse or BP differentials greatly increases the likelihood of acute aortic dissection; in fact, the presence of all three clinical features results in Pos LR of 66.0 (95% CI 4.1-1062.0) (2).
2)What is the most likely diagnosis? Be as specific as possible.
This is a Stanford Type A Aortic Dissection as it involves the ascending aorta. There are a few ways to classify aortic dissections (3). The simplest way is to utilize the Stanford classification which categorizes aortic dissections into two groups based on whether or not the ascending aorta is involved; type A involves the ascending aorta, whereas type B does NOT. This is clinically very useful as management differs depending on whether the ascending aorta is involved. In the setting of an acute aortic dissection, simply looking to see if the ascending aorta is involved can greatly expedite appropriate care, as the management of type A aortic dissections is primarily surgical (3,4,5,7).
3)What are some other imaging modalities that you can use to make the diagnosis?
There are several options available to the emergency physician confronted with a patient suspected of having an acute aortic dissection including plain film chest radiography, echocardiography (transthoracic and transesophageal), CT, MR, and angiography (5,6). Each imaging modality has its advantages and disadvantages depending on the acuity of the clinical presentation and availability of local resources. Let’s start out with plain films. The chest X-ray may demonstrate an abnormality ~88% of the time (7). Widened mediastinum is seen in 60% of cases and the absence of this finding yields a negative LR of 0.3 (95% CI 0.2-0.4) (7). Other plain film abnormalities include pleural effusions which are present in 16% of cases (95% CI 12-21%) and displaced intimal calcifications in 9% (95% 6-13%) (1). In most instances, CT of the thoracic aorta with intravenous contrast is the go-to test in most centers (5,6,7). CT is relatively quick in terms of image acquisition and accurate with sensitivity and specificity near 100% and 98%, respectively (7). MR is also a highly sensitive and specific imaging modality; however, it involves transferring a potentially unstable patient to an MRI suite, away from the close monitoring and resuscitation capabilities of the emergency department. Also, it reasons that any significant dissection would be picked up on CT, a test easier to perform. Bedside echocardiogram is an option, and although it has several limitations, it has definite potential advantages. These include the ability to perform a rapid bedside assessment and the ability to detect some of the complications of dissection including pericardial tamponade, acute aortic insufficiency, and regional wall motion abnormalities (6,7). Transthoracic echo is limited to viewing the ascending aorta and proximal arch; however, if an intimal flap is detected, it is helpful in the detection of type A dissections (7). In fact, transthoracic echo is far more sensitive at detecting type A than type B dissections with reported sensitivity of 78-100% for type A compared to 31-55% for type B (6). Transesophageal echo is highly sensitive at detecting dissections with sensitivity and specificity of 99% and 89%, respectively (6). However, the technique is highly operator-dependent and requires esophageal intubation as well as sedation to prevent any hypertensive response to the procedure, making this a secondary option (6).
4)What is the appropriate ED management?
When it comes to managing patients with acute aortic dissection in the emergency department, the main goal is to reduce aortic wall stress and shear force as these can further extend the dissection (7,8,9). The propagation of the dissection with the subsequent risk of aortic rupture is a leading cause of mortality in these patients (9). As always, attention is directed at ensuring airway, breathing, and circulation. However, in the setting of aortic dissection our main emphasis is all about keeping the blood within the true lumen of the vascular tree. Intravenous access is required to administer intravenous drugs that can maintain the heart rate at approximately 60 beats per minute and the systolic blood pressure at approximately 100-120 mmHg (7). There are several medication options. IV beta-blockers such as esmolol and labetalol are commonly used; calcium channel blockers such as diltiazem and verapamil can be used as well (7). Vasodilators such as nicardipine, nitroprusside, and nitrogycerin can be used; however, the heart rate must be controlled first with beta blockers or calcium channel blockers to prevent a reflex tachycardia. This reflex tachycardia may increase shear forces on an already compromised aortic intima making matters worse (7). While all of this is being done and the patient is being stabilized, consultation with a cardiothoracic or vascular surgeon should be emergently obtained to ensure coordination of care; these patients are going to one of two places – the operating room or the intensive care unit.
Take Home Points
- Chest pain is a high-risk, no-nonsense chief complaint. Consider a differential that is relevant to the emergency department and don’t forget about acute aortic dissection.
- History and physical exam is largely not enough to confidently rule in or rule out aortic dissection. However, the constellations of findings in a chest pain patient (e.g. acute onset, abrupt chest pain described as sharp/tearing/ripping pain radiating into the back, with exam findings notable for BP and/or pulse differentials with focal neurological deficits) greatly increases the pretest probability of aortic dissection, so look for these in the H and P.
- Imaging options beyond just plain radiographs include TEE, TTE, CT angiography, and MR. The usual go-to is the CT as it has the advantages of being relatively rapid with high sensitivity and specificity. MR is usually not a great choice as it takes too long and creates a potentially unsafe situation for the patient. Echo, either TTE or TEE, can be performed at the bedside. TEE is a much better test, but is limited by the need for sedation, whereas TTE is much less sensitive, but is easier to perform and can still pick up an obvious type A dissection.
- Management is all about the ABCs and reducing aortic shear forces with the use of IV beta-blockers, calcium channel blockers and vasodilators. The target HR is approximately 60 bpm, and the SBP goal range is 100-120 mmHg. Appropriate surgical and critical care teams should be notified, as this patient is going to the OR or the ICU.
References
- Woo, KC and Schneider JI. High-Risk Chief Complaints I: Chest Pain-The Big Three. Emergency Medicine Clinics of North America. 2009;685-712.
- Klompas, M. Does This Patient have an Acute Thoracic Aortic Dissection? 2002;287:2262-2272.
- Golledge, J and Eagle, KA. Acute Aortic Dissection. The Lancet. 2008;372:55-66.
- Harrington, PB, Davies, JE, Melby, SJ. Diagnosis and clinical management of aortic dissection. Research Reports in Clinical Cardiology. 2014;5:123-132.
- Hurley, KF, and Ducharme, J. The utility of multiple imaging modalities to diagnose acute aortic dissection. 2008;10(1):75-80.
- Baliga, RR et al. The Role of Imaging in Aortic Dissection and Related Syndromes. J Am Coll Cardiology. 2014;7(4):406-424.
- Lo, BM. An Evidence Based Approach to Acute Aortic Syndromes. Emergency Medicine Practice. 2013;15(12):1-24.
- Erbel, R, Aboyans, V, Boileau, C et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). European Heart Journal.2014;35: 2873-2926.
- Diercks, DB, Promes, SB, et al. ACEP Clinical Policy: Critical Issue in the Evaluation and Management of Adult Patients With Suspected Acute Nontraumatic Thoracic Aortic Dissection. Annals of Emergency Medicine. 2015;65(1):32-42.
ray
Latest posts by ray (see all)
- Temper Tantrums Gone Wrong – Boxer’s Fracture - March 4, 2017
- More than just a fracture! - December 21, 2016
- ST Elevation in aVR – The Widow Maker - November 10, 2016



0 Comments