Case:
A 48-year-old man with no PMH presents via EMS with dyspnea and diarrhea worsening over 2-3 weeks. He has attempted to self-treat the diarrhea with loperamide. He also reports generalized weakness, swelling in both his legs, and palpitations.
PMH: Questionable COPD – treated once
PSH: denies
Meds: denies
Allergies: NKDA
SH: former smoker, no recent travel
PE:
VS: HR 157, RR 26, BP 188/125, temp 98.8 and Sat 98% on NC.
Gen: Anxious-appearing male
HEENT: NCAT, PERRL, dry mucus membranes
Neck: +JVD
CV: tachycardic, irregularly irregular
Resp: decreased breath sounds at bases with b/l rales, R>L
Abd: Soft, NT/ND, no mass
GU: significant scrotal edema
Ext: diffuse b/l lower extremity edema up to thighs
Skin: excoriated skin rash on abdomen and extremities
Neuro: AAOx3, no motor deficits
Initial interventions
An IV catheter and monitor were placed. NS 500ml bolus and 15mg IV diltiazem were administered. EKG demonstrated A-fib in the 150’s, and CXR showed a left lower lobe consolidation with effusion. Bedside sono revealed diffuse B-lines.
Labs:
VBG: 7.43/39.7/37.7, BE -2, lactate 2.9

TSH: <0.05
Free T4: 18.7
Troponin: <0.02
BNP: 686
UA: trace ketones, small protein and 300 glucose
UTox: negative
ED Course:
Patient received empiric antibiotic coverage with vancomycin and piperacillin/tazobactam, 2L of NS, 30 mg of PO propranolol, 20 mg PO methimazole, and 100 mg IV hydrocortisone. He became hypotensive to 74/40 during ED course and central line access was attempted. A right internal jugular central venous catheter was then placed for infusion of norepinephrine. Endocrinology consult recommended adding cholestyramine, steroids, and potassium iodide (SSKI).
The patient was then admitted to the MICU for further management of thyroid storm complicated by atrial fibrillation, heart failure, and coagulopathy.
Thyroid Storm
Pathology
Thyroid storm involves the dysregulation of thyroid hormone and its downstream effects. The hypothalamus secretes thyrotropin-releasing hormone (TRH), which in turn, stimulates the release of thyroid stimulating hormone (TSH) by the anterior pituitary gland. TSH binds to the thyroid gland, initiating iodide uptake into the thyroid follicular cells, which is then oxidized and organified by thyroid peroxidase (TPO). Tyrosine residues on thyroglobulin is then iodinated by TPO, forming thyroxine (T4) and small amounts of triiodothyronine (T3). T4 can be converted in the periphery to T3. Peripheral levels of T3 and T4 then create a negative feedback loop to regulate levels of TRH and TSH. Intracellularly, T3 and T4 induce gene activation in the nucleus, resulting in tissue thermogenesis, increased basal metabolic rate, proliferation of adrenergic receptors, and reduced systemic vascular resistance (1,2).
Most commonly, thyroid storm is caused by a primary thyroid problem due to uninhibited production of T4 despite a low TSH. Graves’ disease is the most likely culprit, but destructive thyroiditis and toxic multinodular goiter can also be etiologies of primary hyperthyroidism. Although rare, thyroid storm may also be due to a secondary hyperthyroidism where the thyroid gland is being stimulated to increase T4 levels, such as in a TSH-secreting pituitary adenoma or b-hCG-secreting hydatidiform mole (if you recall from medical school, TSH shares the alpha subunit with hCG, LH, and FSH). Stressors, such as major burns, infection, myocardial ischemia, or trauma can precipitate dysregulation of the thyroid axis and subsequently, thyroid storm (2,3)
Diagnosis
Thyroid storm is a clinical diagnosis of increased end organ effects from thyroid hormone. The Burch-Wartofsky score (BWS) can be helpful to differentiate between a hyperthyroid state and thyroid storm.
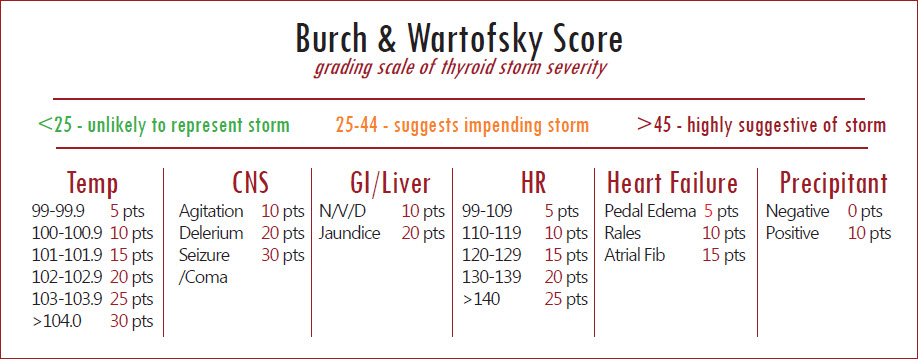
Obtained from http://www.tamingthesru.com/blog/grand-rounds/2016/5/25/grand-rounds-recap-525
The BWS does not incorporate any laboratory studies. The Japanese Thyroid association is very similar but incorporates increased levels of thyroid and requires a diagnosis of hyperthyroidism for inclusion (3).

Japan Thyroid Association diagnostic criteria for thyroid storm (3)
Treatment
Treatment involves stepwise blocking at many different points of the thyroid pathway. Blocking of end organ effects with a beta-receptor antagonism is important. Propranolol is often cited as the preferred beta blocker because it can block peripheral conversion of T4 to T3. In critically ill patients, using a short-acting agent such as esmolol may be ideal in case of decompensation (more on this later). The next step is to block the production of thyroid hormone with thionamides, such as PTU or methimazole, which inhibit TPO. Steroids (dexamethasone or hydrocortisone) can be used to prevent the conversion of T4 to T3. One hour after administration of thionamides, you may give iodine in the form of Lugol’s solution or potassium iodide to prevent further release of preformed thyroid hormone and reduce transportation and oxidation of iodide. Finally, cholestyramine works by preventing the reuptake of thyroid hormone in the intestine during its enterohepatic circulation and can be given as an adjunct (1,2,3).
One of the most challenging aspects of this case was the management of heart failure in the setting of thyrotoxicosis, atrial fibrillation with rapid ventricular rate (RVR), and possible pneumonia.
High Output Heart Failure
High output heart failure (HOHF) is defined as a low systemic vascular resistance combined with increased cardiac output. There are numerous causes of high output heart failure, including chronic anemia, chronic hypercapnia, thyrotoxicosis, sepsis, beriberi, pregnancy, obesity, liver disease, carcinoid syndrome, systemic arteriovenous (AV) fistula, Paget’s disease, multiple myeloma, and Albright’s disease (4). A 15-year review at the Mayo Clinic of patients with confirmed HOHF based upon clinical signs and right heart catheterization found 130 cases: nine were as a result of anemia, and one was due to thyrotoxicosis. Attempts were made to exclude all patients with clear etiologies of HOHF. Of the remaining 120 patients, etiologies in order of most common were (5):
- Morbid obesity
- Arteriovenous shunts
- Cirrhosis
- Pulmonary disease (COPD, connective tissue disorders, interstitial lung disease, bronchiectasis, and bronchiolitis obliterans)
- Myeloproliferative hematologic disorders
Eventually, all etiologies of HOHF result in AV shunting or decreased systemic vascular resistance. This, in turn, leads to decreased systemic arterial blood pressure and sympathetic neural activation and increased cardiac output and activation of the renin-angiotensin-aldosterone system. Together, these changes cause salt and water retention, leading to clinical signs of decompensated systolic heart failure. Properly differentiating between the different subtypes of heart failure is difficult in the ED. Assessment begins with checking vital signs, identifying symptoms and clinical exam findings consistent with fluid overload, and bedside echocardiography.
| SVR | EF | CO | Overload | |
| HOHF | Low | Normal | High | Yes |
| HFpEF | High | Normal | Low | Varies |
| HFrEF | High | Low | Low | Yes |
Table 1: SVR – systemic vascular resistance; EF – ejection fraction; CO – cardiac output; overload – clinical signs of fluid overload; HFpEF – heart failure with preserved ejection fraction; HFrEF – heart failure with reduced ejection fraction
HOHF may be differentiated from heart failure with reduced ejection fraction (HFrEF) by the presence of warm extremities, preserved EF, mixed venous oxygen saturation >70%, and a likely underlying etiology. Definitive treatment is aimed at the underlying cause of the HOHF, be it correction of anemia, thiamine repletion, infection control, weight loss, or treatment of thyrotoxicosis. For the treatment of HOHF itself, there is no good data to guide management, but patients with overt hypotension may require vasopressor support and salt/water restriction until the underlying etiology is adequately corrected (4,5).
Esmolol for atrial fibrillation
Atrial fibrillation in critically ill patients requires close bedside monitoring and frequent reassessment. Esmolol is a beta-1 selective beta-receptor antagonist with a short half-life, allowing for titration of the medication to desired effect and when discontinued, recovery from beta-blockade within 10-20 minutes (6,7,8). It is renally excreted but its metabolite is 1000x weaker than the active drug, thus renal failure will not result in any clinically relevant effects (8). These properties make it an excellent agent for patients with borderline hypotension or other critically ill patients. As with all beta-blockers in atrial fibrillation, it is not recommended in combination with intravenous calcium channel blockers or other medications that may cause bradycardia or hypotension (6). It should be dosed with a 500 mcg/kg bolus over 1 minute, then 50mcg/kg/min infusion. Afterwards, it can be titrated up every 4 minutes by re-bolusing 500 mcg/kg and then increasing the infusion by 50 mcg/kg/min increments up to a maximum of 250-300 mcg/kg/min (6).
A single center retrospective review of 107 post-CABG patients with atrial fibrillation with RVR by Hilleman et al found that significantly more patients reverted to sinus rhythm or achieved rate control <90 bpm with esmolol than with diltiazem. In addition, significantly fewer adverse effects (heart failure, hypotension, AV block, and bradycardia) and fewer discontinuations due to these adverse effects were ascribed to esmolol. Of the 26 cases of adverse effects for esmolol, 14 were managed simply by reducing the dosage of the medication (9).
An older, small (n=45), randomized, open label study compared esmolol to verapamil in the acute treatment of atrial fibrillation/flutter with RVR in otherwise stable patients. The study utilized a peculiar stepwise titration scheme for both medications. Both groups had a reduction in baseline ventricular rate from approximately 140 bpm to approximately 100 bpm over a period of 30 minutes. Half of the 14 patients with new onset atrial fibrillation or flutter in the esmolol group converted to sinus rhythm compared to two of the 17 patients on verapamil by the end of the study. Similar proportions in both groups experienced hypotension (systolic blood pressure <90 mm Hg), with similar proportions requiring intervention. However, it should be noted that this study administered esmolol in fixed doses, ignoring differences in body weight (10). The paper does not provide individual patient weights, but based upon the range of weights provided and the recommended dosing of esmolol, this scheme likely gave much higher doses of esmolol than is recommended. Thus, had esmolol been given in proper weight based dosing, it is feasible that the rates of adverse effects may have been lower.
Unfortunately, there is a paucity of data examining the use of esmolol in the ED. Most studies evaluating esmolol for atrial fibrillation do so in the postoperative period. Several reviews on the management of atrial fibrillation mention the use of esmolol for rate control based upon experience and its favorable pharmacokinetic profile. Despite its lack of rigorous study in the acute care setting, the European Society of Cardiology also recommends using esmolol when hemodynamic instability is a concern (12).
As further evidence of its utility in critically ill patients, esmolol has been evaluated in a randomized, open-label study of ICU patients with septic shock. It was used to maintain HR less than 95/min and found significantly lower norepinephrine and fluid requirements, 28-day mortality, and various other hemodynamic parameters. (12). A review of beta-blockade in the septic patient by Pemberton et al, proposes several mechanisms for its benefit in these patients, which would likely apply to many other critically ill patients. Sympathetic overstimulation leads to tachycardia, possibly tachycardia-induced dysrhythmias/cardiomyopathies, reduced filling time, increased myocardial oxygen demand, and is associated with worse outcomes. Small trials involving patients with heart failure and traumatic injuries demonstrated reduced proinflammatory cytokines with beta-blockade, suggesting a possible anti-inflammatory component (13). Furthermore, a case-matched study of 3,112 ICU patients by Christensen et al showed a 30-day mortality benefit among patients on preadmission beta blockers that was more pronounced with cardioselective beta-blocks, such as esmolol (14). These studies suggest a role for beta-blockade, specifically for esmolol in a wide range of critically ill patients that warrants further study. In the patient with atrial fibrillation with rapid ventricular rate, esmolol should be considered a first-line medication.
TL;DR
Thyroid storm has a high morbidity and mortality. Thyroid Storm treatment involves blocking at many different points. Treat the underlying cause of high output heart failure. Consider using esmolol for atrial fibrillation with RVR in critically ill patients.
Thank you to Dr. Eric Schnitzer for his help with the post and presenting the material during conference!
References
- Ross Douglas S., Burch Henry B., Cooper David S., Greenlee M. Carol, Laurberg Peter, Maia Ana Luiza, Rivkees Scott A., Samuels Mary, Sosa Julie Ann, Stan Marius N., and Walter Martin A.. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid. October 2016;26(10): 1343-1421.
- Chiha Maguy, Samarasinghe Shanika, Kabaker Adam. Thyroid Storm: An updated review. Journal of Intensive Care Medicine. 2015;30(3):131-140.
- Tetsurou Satoh, Osamu Isozaki, Atsushi Suzuki, Shu Wakino, Tadao Iburi, Kumiko Tsuboi, Naotetsu Kanamoto, Hajime Otani, Yasushi Furukawa, Satoshi Teramukai, Takashi Akamizu. 2016 Guidelines for the management of thyroid storm from The Japan Thyroid Association and Japan Endocrine Society. Endocrine Journal. December 2016;63(12):1025-1064.
- Mehta PA, Dubrey SW. High output heart failure. Q J Med. April 2009;102(4):235-241.
- Reddy YNV, Melenovsky V, Redfield MM, Nishimura RA, Borlaug BA. High-Output Heart Failure: A 15-year experience. Journal of the American college of cardiology. August 2016;68(5):473-482.
- Reynolds RD, Gorczynski RJ, Quon CY. Pharmacology and pharmacokinetics of esmolol. J Clin Pharmacol. Mar 1986;26 Suppl A:A3-A14.
- BREVIBLOC PREMIXED Injection [package insert]; Baxter Healthcare Corporation; Revised 2012.
- Krumpl G, Domanovits H, Stix G, Heinz G. Esmolol in cardiology, emergency, and critical-care medicine. Austrian Journal of Cardiology. 2012;19(Suppl A):2-8.
- Hilleman DE, Reyes AP, Mooss AN, Packard KA. Esmolol versus diltiazem in atrial fibrillation following coronary artery bypass graft surgery. Curr Med Res Opin. 2003;19(5):1-8.
- Platia EV, Michelson EL, Porterfield JK, Das G. Esmolol versus verapamil in the acute treatment of atrial fibrillation or atrial flutter. Am J Cardiol. April 15, 1989;63:925-929
- Camm AJ, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010; 31: 2369–429.
- Morelli A, Ertmer C, Westphal M, Rehberg S, Kampmeier T, Ligges S, Orecchioni A, D’Egidio A, D’Ippoliti F, Raffone C, Venditti M, Guarracino F, Girardis M, Tritapepe L, Pietropaoli P, Mebazaa A, Singer M. Effect of Heart Rate Control With Esmolol on Hemodynamic and Clinical Outcomes in Patients With Septic ShockA Randomized Clinical Trial. JAMA. 2013;310(16):1683-1691.
- Pemberton P, Veenith T, Snelson C, Whitehouse T. Review article: Is it time to beta block the septic patient? BioMed Research International. May 2015. Article ID 424308:6 pages
- Christensen S, Johansen MB, Tønnesen E., et al., “Preadmission beta-blocker use and 30-day mortality among patients in intensive care: a cohort study,” Critical Care. 2011; 15(2):R87
edenkim
Latest posts by edenkim (see all)
- Cracking Skulls: When is Neurosurgical Intervention Helpful for ICH? - October 1, 2017
- Valproic Acid Toxicity – The evidence behind different treatment options - July 5, 2017
- Thyroid Storm – How to Recognize It and How to Treat It - June 4, 2017



1 Comment
Jesus · June 6, 2017 at 9:24 am
Awesome read, Eden! Thanks for the content, brother!